Associate Editor
- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
A case for additive manufacturing
Additive Design in Surgical Solutions (ADEISS) centre helps one med-tech startup bring its spinal clamp design to life.
- By Lindsay Luminoso
- October 1, 2018
- Article
- Metalworking

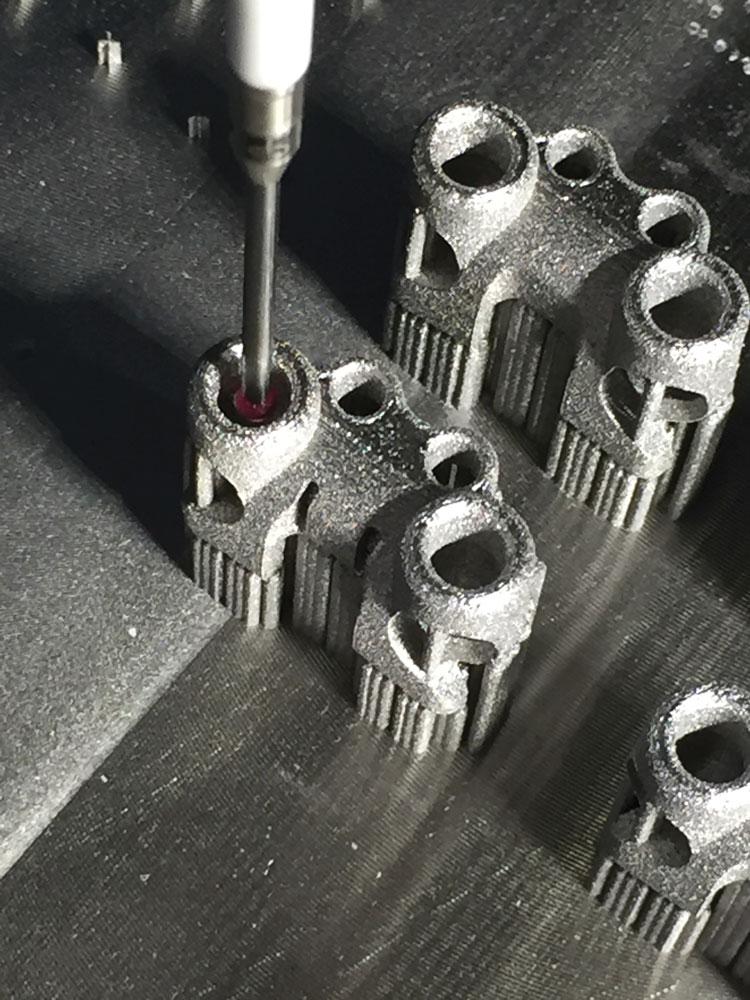
A-Line Orthopaedics turned to ADEISS to develop and 3-D-print its innovative DC2 spinal implant system on a Renishaw AM 400 at its centre in London, Ont.
Additive manufacturing (AM) is having a major impact on the healthcare industry, helping to save and improve lives through medical device innovation. This is particularly true in Canada, thanks to the collaborative expertise of the Additive Design in Surgical Solutions (ADEISS) centre, based in London, Ont. ADEISS, a wholly owned subsidiary of Western University, is working in partnership with Renishaw, Mississauga, Ont., and the London Medical Network to create a first-of-its-kind 3-D printing centre in North America.
“In London, we have the clinical staff from the hospitals, a research component with the university, and a number of world-class research institutes. This site [ADEISS] brings the engineering component. The idea was to base all the components of medical device development in one place,” said Matt Parkes, technical manager at ADEISS. “We have the technical capabilities to speed up the innovation process of new medical devices and produce devices that can actually help patients.”
According to Parkes, the partnership was created to deal with a significant market challenge. Previously there were very few ways for startups and companies to get devices into the market using suppliers in Canada. Typically, a clinician who came up with a novel design for a device would have to license the idea to a med-tech company in the U.S. or use facilities in the U.S. to develop the product. It was rare for a medical device concept to be developed and produced in Canada—until now.
“With ADEISS, we are helping to facilitate design and development in Canada,” added Parkes. This can be seen in action through a recent project helping London-based medtech startup A-Line Orthopaedics develop its innovative spinal implant system, DC2.
AN IDEA IS BORN
The startup began exploring new device options for dealing with problems that occur in orthopaedic surgery, specifically repairing fractures to the C1 and C2 vertebrae—very severe spinal cord injuries. Currently, surgeons fix them by fusing them together using bone screws. The vertebrae’s location and small size make the procedure difficult to perform, and the risk of blood loss is high.
The surgeons and researchers at A-Line came up with an idea to use a clamping mechanism on the vertebrae rather than a bone screw, so there is less risk of damaging a blood vessel.
The DC2 spinal implant system features a novel jaw design that uses spikes to hold onto the bone. The jaws are actuated using a ball joint, much like a hip joint, so that the device can be put into the patient and the jaws can all close along the same path. Parkes explains that this device lets the surgeon make a much smaller incision than previous methods would allow, which reduces recovery time and complications for the patient.
“Overall, we think this approach is better for surgeons and patients,” Parkes added. And because not everyone’s vertebrae are the same, this ball joint allows the clamp to articulate and pivot to apply the needed clamping force. The design also needed to include many fine features, as the device measures 25 by 20 by 12 millimeters. It has an articulated joint that moves the clamping jaw, but required testing and verification to ensure proper mechanical movement before the startup could bring the device to market. That’s where ADEISS came in.
“We were able to collaborate straight away with the startup,” said Parkes. “They were able to provide us with all the clinical expertise, while we provided the technical capability.”
SUBTRACTIVE MANUFACTURING VERSUS AM
Right out of the gate, ADEISS recognized that the very fine features were incompatible with traditional machining methods, which would require production of several components that would need to be assembled.

The DC2 functional prototypes are printed in a medical grade titanium alloy, Ti6Al4V, and include a number of very fine features.
This would prove both time-consuming and costly, especially considering the need for tooling and setup adjustments. AM proved to be the right method for the job. The team used a Renishaw AM 400 laser powder bed 3-D printer to develop functional prototypes using a medical grade titanium alloy, Ti6Al4V, and integrated a number of very fine features. They then tested many design iterations to ensure the device had equal or better mechanical properties compared with the existing procedure.
Parkes explained that Ti6Al4V has been used successfully in the production of medical devices, including by subtractive manufacturing.
“We were able to create a part design that was suitable for 3-D printing,” Parkes added. “It is all one piece and was designed in such a way that it can be fixed on a build plate to allow for subtractive machining on some key interfaces. Because we had no fixed setup, we were able to use 3-D printing to evolve the design with little additional costs.”
The cost to produce the component using AM was extremely competitive with the cost of subtractive machining. Parkes added that this isn’t always the case, especially when it comes to medical design.
“Our expertise is really in how best to utilize 3-D printing and design for AM,” said Parkes. “We need to consider aspects like orienting the part and meeting tight tolerances, while being efficient and economical enough to produce parts with a small initial volume.”
Medical devices, especially ones designed for extremely delicate internal structures, need to meet strict tolerances. The metal ball features have specific tolerances that needed to be met to ensure the part could function.
These features were inspected using a coordinate measuring machine and computed topography at Expanse Microtechnologies, Toronto. However, AM allowed for artifacts to be included in every build for quick in-process checks to ensure accuracy. The team also visually inspected the components and ensured process health by looking at part density. According to ADEISS, metal parts fabricated with selective laser melting are routinely measured within 0.5 per cent of the density of conventionally manufactured parts.
NEXT STEP
Parkes explained that this project has moved rather quickly from when A-Line first began brainstorming to ADEISS getting involved to the completion of a functional prototype.
The next step for ADEISS is to get the regulatory approvals to allow projects like this to be taken all the way through to production. The partnership is hoping to attain its ISO 13485 medical quality standards by the end of the calendar year.
Combining clinical and manufacturing expertise under one roof has made it easier to design and develop state-of-the-art medical innovations, and 3-D printing is a huge part of that push forward.

The one-piece device was designed so it can be fixed on a build plate to allow for subtractive machining on some key interfaces.
“We are able to bridge the gaps in any problem area, whether in the basic science, research or clinical practice stage,” said Parkes. “3-D printing is really emerging on the forefront, and certainly ADEISS is looking to advance 3-D printing in the healthcare environment. Once we pass the regulatory standards, we can begin producing parts in a quality-controlled regulatory setting. We can translate novel concepts all the way from concept to helping patients. Innovation is needed in the medical industry, and 3-D printing has a key role to play in that.”
Associate Editor Lindsay Luminoso can be reached at lluminoso@canadianmetalworking.com.
A-Line Orthopaedics, www.alineorthopaedics.com
ADEISS, www.adeiss.ca
Renishaw Canada, www.renishaw.com
About the Author

Lindsay Luminoso
1154 Warden Avenue
Toronto, M1R 0A1 Canada
Lindsay Luminoso, associate editor, contributes to both Canadian Metalworking and Canadian Fabricating & Welding. She worked as an associate editor/web editor, at Canadian Metalworking from 2014-2016 and was most recently an associate editor at Design Engineering.
Luminoso has a bachelor of arts from Carleton University, a bachelor of education from Ottawa University, and a graduate certificate in book, magazine, and digital publishing from Centennial College.
Related Companies
subscribe now


Keep up to date with the latest news, events, and technology for all things metal from our pair of monthly magazines written specifically for Canadian manufacturers!
Start Your Free Subscription- Trending Articles
Automating additive manufacturing

CTMA launches another round of Career-Ready program

Collet chuck provides accuracy in small diameter cutting

Sandvik Coromant hosts workforce development event empowering young women in manufacturing

GF Machining Solutions names managing director and head of market region North and Central Americas

- Industry Events
MME Winnipeg
- April 30, 2024
- Winnipeg, ON Canada
CTMA Economic Uncertainty: Helping You Navigate Windsor Seminar
- April 30, 2024
- Windsor, ON Canada
CTMA Economic Uncertainty: Helping You Navigate Kitchener Seminar
- May 2, 2024
- Kitchener, ON Canada
Automate 2024
- May 6 - 9, 2024
- Chicago, IL
ANCA Open House
- May 7 - 8, 2024
- Wixom, MI